Introduction
General Information
Mass spectrometry (MS) is an essential analytical technique for high-throughput analysis in proteomics and metabolomics. The development of new separation techniques, precise mass analyzers and experimental protocols is a very active field of research. This leads to more complex experimental setups yielding ever increasing amounts of data. Consequently, analysis of the data is currently often the bottleneck for experimental studies. Although software tools for many data analysis tasks are available today, they are often hard to combine with each other or not flexible enough to allow for rapid prototyping of a new analysis workflow.
OpenMS, a software framework for rapid application and method development in mass spectrometry has been designed to be portable, easy-to-use, and robust while offering a rich functionality ranging from basic data structures to sophisticated algorithms for data analysis (https://www.nature.com/articles/s41592-024-02197-7).
Ease of use: OpenMS follows the object-oriented programming paradigm, which aims at mapping real-world entities to comprehensible data structures and interfaces. OpenMS enforces Coding Conventions that ensure consistent names of classes, methods and member variables which increases the usability as a software library. Another important feature of a software framework is documentation. We decided to use doxygen to generate the class documentation from the source code, which ensures consistency of code and documentation. The documentation is generated in HTML format making it easy to read with a web browser.
Robustness: Robustness of algorithms is essential if a new method will be applied routinely to large scale datasets. Typically, there is a trade-off between performance and robustness. OpenMS tries to address both issues equally. In general, we try to tolerate recoverable errors, e.g. files that do not entirely fulfill the format specifications. On the other hand, exceptions (usually interally derived from BaseException) are used to handle fatal errors. To check for correctness, more than 1000 unit tests (see How To Write Tests) are implemented in total, covering public methods of classes. These tests check the behavior for both valid and invalid use. Additionally, preprocessor macros are used to enable additional consistency checks in debug mode, enforce pre- and post-conditions, and are then disabled in productive mode for performance reasons.
Extensibility: Since OpenMS is based on several external libraries it is designed for the integration of external code. All classes are encapsulated in the OpenMS namespace to avoid symbol clashes with other libraries. Through the use of C++ templates, many data structures are adaptable to specific use cases. Also, OpenMS supports standard formats, such as mzML and mgf, and is itself open-source software. The use of standard formats ensures that applications developed with OpenMS can be easily integrated into existing analysis pipelines. OpenMS source code is released under the permissive BSD 3 license and hosted on https://github.com/OpenMS/OpenMS. This allows users to participate in the project and to contribute to the code base.
Scriptable: OpenMS allows exposing its functionality through python bindings (pyOpenMS). This eases the rapid development of algorithms in Python that later can be translated to C++. Please see our pyOpenMS documentation for a description and walk-through of the pyOpenMS capabilities.
Portability: OpenMS supports Windows, Linux, and MacOS X platforms.
The structure of the OpenMS Framework
The following image shows the overall structure of OpenMS:
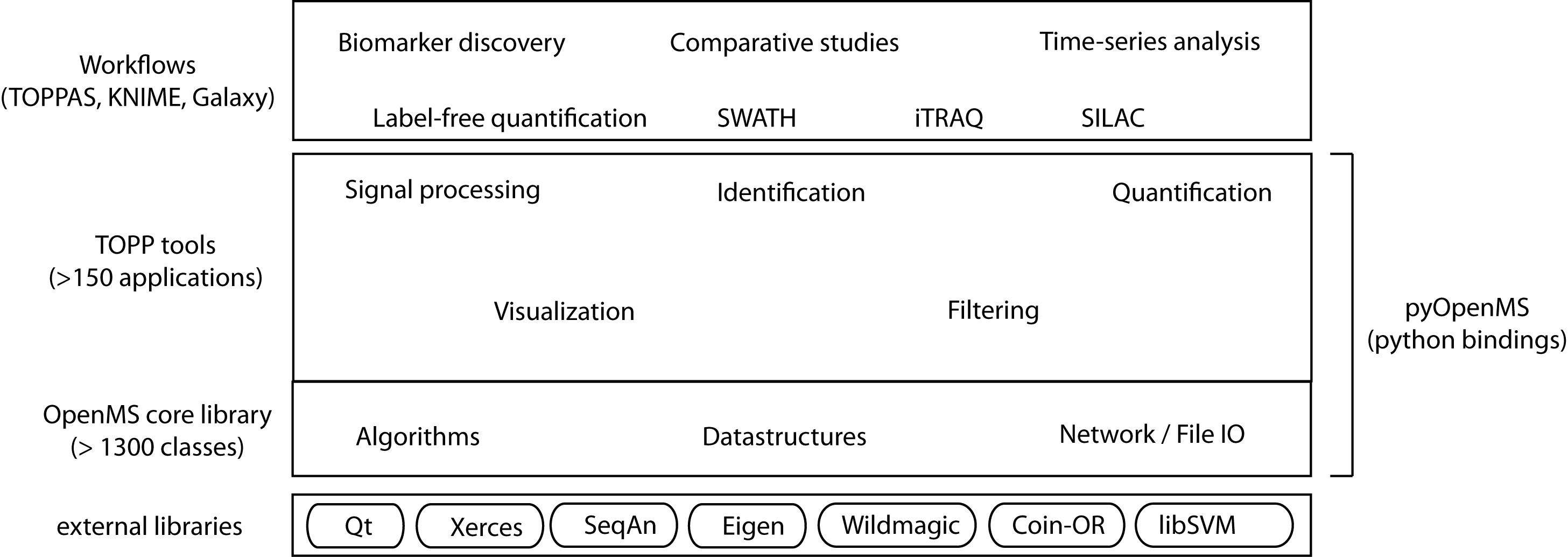
Overall design of OpenMS. Kindly provided by Timo Sachsenberg.
The structure of the OpenMS framework.
The OpenMS software framework consists of three main layers:
- OpenMS Library: the object-oriented OpenMS core library contains over 1,300 classes and is built on modern C++ infrastructure with native compiler support on Windows, Linux and OS X. The classes are representing core concepts in mass spectrometry as well as the corresponding ontologies defined by the Human Proteome Organization Proteomics Standard Initiative (HUPO-PSI).
- Scripting: a well-defined Python API offers scripting for rapid software prototyping and interactive data exploration by researchers with advanced scripting skills. The pyOpenMS interactive Python interface, providing easy integration of the OpenMS library with other scientific Python libraries.
- TOPP tools: The TOPP documentation["The %OpenMS PiPeline (TOPP)"] is a set of executables covering most core tasks in computational mass spectrometry and has grown beyond proteomics applications and now includes Metabolomics and others. These tools are created using algorithms from the OpenMS library. These tools form the building blocks of complex workflows.
- Workflow: a set of over 185 different tools for common mass spectrometric tasks can be accessed by routine users through workflow systems, such as KNIME or Galaxy, the OpenMS-specific TOPPAS tutorial["TOPPAS"], or even custom bash scripts.
Each level of increasing abstraction provides better usability, but limits the extensibility as the Python and workflow levels only have access to the exposed Python API or the available set of TOPP tools respectively. Increasing abstraction, however, makes it easier to design and execute complex analyses, even across multiple omics types. By following a layered design the different needs of bioinformaticians and life scientists are addressed.
Developing with OpenMS
Before we get started developing with OpenMS, we would like to point to some information on the development model and conventions we follow to maintain a coherent code base.
Development model
OpenMS follows the Gitflow development workflow which is excellently described here. Additionally we encourage every developer (even if he is eligible to push directly to OpenMS) to create his own fork. The GitHub people provide superb documentation on forking and how to keep your fork up-to-date. With your own fork you can follow the Gitflow development model directly, but instead of merging into "develop" in your own fork you can open a pull request. Before opening the pull request, please check the checklist.
Some more details and tips are collected here.
Conventions
See the manual for proper coding style: Coding Conventions, also see: C++ Guide.
Commit Messages
In order to ease the creation of a CHANGELOG we use a defined format for our commit messages. See the manual for proper commit messages: How to write Commit messages.
Automated Unit Tests
Pull requests are automatically tested using our continuous integration platform. In addition we perform nightly test runs covering different platforms. Even if everything compiled well on your machine and all tests passed, please check if you broke another platform on the next day. Nightly tests: CDASH
Experimental Installers
We automatically build installers for different platforms. These usually contain unstable or partially untested code - so use them at your own risk. The nightly (unstable) installers are available here.
Technical Documentation
Documentation of classes and tools is automatically generated using doxygen: See the documentation for HEAD See the documentation for the latest release branch
Building OpenMS
Before you get started coding with OpenMS you need to build it for your operating system. Please follow the build instructions from the documentation.
Building OpenMS on GNU/Linux
Building OpenMS on Mac OS X
Building OpenMS on Windows
Note that for development purposes, you might want to set the CMake variable CMAKE_BUILD_TYPE to Debug for single configuration generators, such as make or ninja. Otherwise, the default Release will be applied and disables pre-condition and post-condition checks, and assertions.
Choice of an IDE
You are, of course, free to choose your favorite (or even no) IDE for OpenMS development but given the size of OpenMS, not all IDEs perform equally well. We have good experiences with Qt Creator on Linux and Mac, because it can directly import CMake Projects and is rather fast in indexing all files. On Windows, Visual Studio is currently the preferred solution. Additionally, you may want to try JetBrains CLion (it is free for students, teachers and open source projects). Another option is Eclipse with C++ support, which can also import CMake projects directly with the respective CMake generator.
Mass spectrometry terms
The following terms for MS-related data are used in this tutorial and the OpenMS class documentation:
- Raw or profile peak: a typically Gaussian shaped mass peak measured by the instrument.
- Centroid or picked peak: a single m/z, intensity pair as obtained after using a peak picking (also: peak centroiding) algorithm.
- Spectrum / Scan: a mass spectrum containing profile or centroided peaks (profile spectrum) or centroided peaks (peak spectrum). E.g. a low resolution profile (blue) and a centroided peak spectrum (pink) are shown in the figure below.
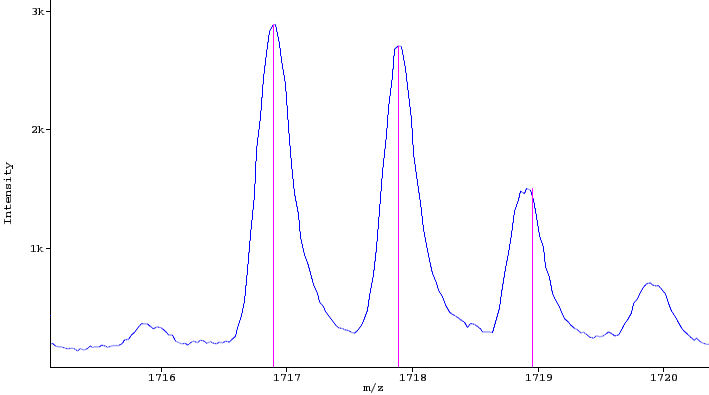
Part of a raw spectrum (blue) with three peaks (red)
- (Peak or Raw) Map: a collection of spectra of a single LC-MS run. If spectra are recorded in profile mode, we usually use the term raw map. If spectra are already centroided we usually refer to them as peak map.
- Feature: a signal from a chemical entity detected in an HPLC-MS experiment, typically a peptide.
The image below shows a peak map and the red circle highlights a feature.
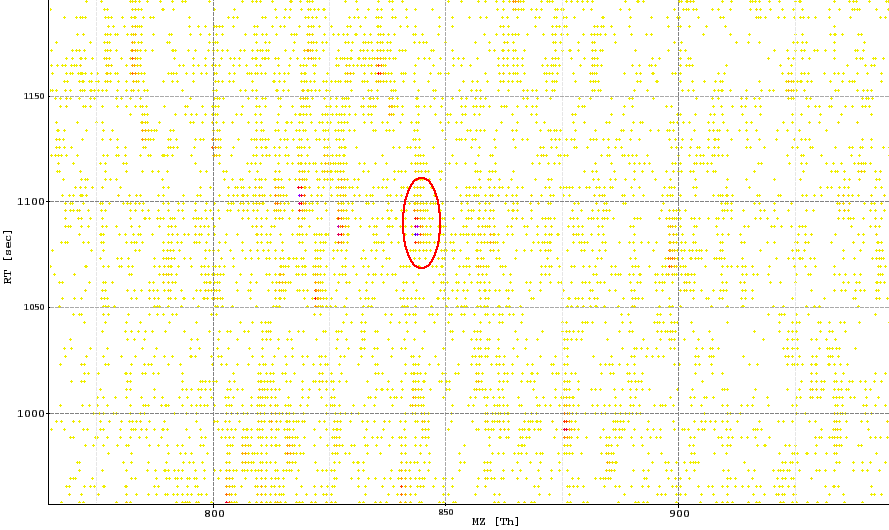
Peak map with a marked feature (red)
OpenMS Library
The extensible OpenMS library implements common mass spectrometric data processing tasks through a well defined API in C++ and Python using standardized open data formats.
Overview on Central Algorithms and Methods
OpenMS provides algorithms in many fields of computational metabolomics and proteomics.
The following list is intended to algorithm and tool developers a starting point to tools and classes relevant to their scientific question at hand. It does not include third-party tools but only tools that were implemented in OpenMS.
- Proteomics:
- Signal processing:
- Conversion from profile to centroided spectra (Tool PeakPickerHiRes)
- Precursor mass correction (Tool HiResPrecursorMassCorrector)
- Filtering:
- Large number of basic filters applicable to different types of data (e.g., remove identified spectra, filter MS2, extract m/z ranges, … in Tool FileFilter and IDFilter)
- Identification:
- Database search:
- Peptides (Tool SimpleSearchEngine and its classes - started simple but is, by now, rather complete peptide identification engine)
- Protein-Protein cross-links (Tool OpenPepXL)
- DeNovo:
- Tool CompNovoCID and its classes
- Quantification:
- Peptide Feature Detection:
- Untargeted, label-free (Tools FeatureFinderCentroided, FeatureFinderMultiplex, and its classes)
- ID-based label-free (Tool FeatureFinderIdentification “new”)
- SILAC-labeling (Tool FeatureFinderMultiplex)
- iTRAQ/TMT (Tool IsobaricAnalyzer)
- Dynamically labeled (SIP) peptides (Tool MetaProSIP)
- Retention Time Alignment:
- Linear map alignment (Tool MapAlignerPoseClustering)
- (Non-)linear map alignment (Tool MapAlignerIdentification “new”)
- Peptide Feature linking (matching of features between runs):
- fast, KD-tree based linking (Tool FeatureLinkerUnlabeledKD)
- QT based clustering and linking (Tool FeatureLinkerUnlabeledQT)
- Protein inference:
- Protein Quantification:
- Targeted data extraction:
- Analysis of data-independent acquisition or SWATH-MS data (Tool OpenSWATH)
- Misc:
- Theoretical spectra generators
- Metabolomics:
- Quantification:
- Small molecule feature detection:
- Untargeted, label-free (Tool FeatureFinderMetabo)
- Retention Time Alignment:
- Linear map alignment (Tool MagAlignerPoseClustering)
- Small molecule feature linking:
- QT based clustering and linking (Tool FeatureLinkerUnlabeledQT)
- fast, KD-tree based linking (Tool FeatureLinkerUnlabeledKD)
- Adduct decharging:
- Linear programming based determination of small molecule ion adducts and charges (Tool MetaboliteAdductDecharger)
- Targeted data extraction:
- Analysis of data-independent acquisition or SWATH-MS data (Tool OpenSWATH)
- Identification:
- Spectral library search:
- Tool MetaboliteSpectralMatcher
- Accurate mass search:
- De novo identification:
- General:
- Mass decomposition algorithms
- Isotope pattern generators
- Quality control (Tools QualityControl) metrics and file format (mzQC and its predecessor QcML)
Directory structure of src folder (/src)
| Folder | Description |
| openms | Source code of core library |
| openms_gui | Source code of GUI applications (e.g.: TOPPView) |
| topp | Source code of (stable) OpenMS Tools |
| util | Source code of (experimental) OpenMS Tools |
| pyOpenMS | Source files providing the python bindings |
| tests | Source code of class and tool tests |
Directory structure of core library (/src/openms/include/OpenMS)
| Folder | Description |
| ANALYSIS | Source code of high-level analysis like PeakPicking, Quantitation, Identification, MapAlignment |
| APPLICATIONS | Source code for tool base and handling |
| CHEMISTRY | Source code dealing with Elements, Enzymes, Residues/Amino Acids, Modifications, Isotope distributions and amino acid sequences |
| COMPARISON | Different scoring functions for clustering and spectra comparison |
| CONCEPT | OpenMS concepts (types, macros, ...) |
| DATASTRUCTURES | Auxiliary data structures |
| FILTERING | Filter |
| FORMAT | Source code for I/O classes and file formats |
| INTERFACES | Interfaces (WIP) |
| KERNEL | Core data structures |
| MATH | Source code for math functions and classes |
| METADATA | Source code for classes that capture metadata about a MS or HPLC-MS experiment |
| SIMULATION | Source code of MS simulator |
| SYSTEM | Source code for basic functionality (file system, stopwatch) |
| TRANSFORMATIONS | Feature detection (MS1 label-free and isotopic labelling) and PeakPickers (centroiding algorithms) |
Within the ANALYSIS folder, you can find several important tools
Directory structure of the algorithmic part of the library (/src/openms/include/OpenMS/ANALYSIS)
| Folder | Description |
| DECHARGING | Algorithms for de-charging (charge analysis) for peptides and metabolites |
| DENOVO | Algorithms for "de-novo" identification tools including CompNovo |
| ID | Source code dealing with identifications including ID conflict resolvers, metabolite spectrum matching and target-decoy models |
| MAPMATCHING | Algorithms for retention time correction and feature matching (matching between runs) |
| MRM | Algorithms for MRM Fragment selection |
| OPENSWATH | OpenSWATH algorithms for targeted, chromatogram-based analysis of MRM, SRM, PRM, DIA and SWATH-MS data |
| PIP | Peak intensity predictor |
| QUANTITATION | Algorithms for quantitative analysis including isobaric labelling |
| RNPXL | Algorithms for RNA cross-linking |
| SVM | Algorithms for SVM |
| TARGETED | Algorithms for targeted proteomics (MRM, SRM) |
| XLMS | Algorithms for Cross-link mass spectrometry |
For the sake of completeness you will find a short list of the THIRDPARTY tools, which are integrated via wrappers into the OpenMS framework (usually called -Adapter e.g. CometAdapter)
Wrapper to third-party tools:
- Search Engines (MSGFPLUS, Comet, ...)
- Protein Inference (Fido)
- Spectral Library Search (SpectraST)
- Metabolite Identification (Sirius)
- Score calibration and FDR calculation (Percolator)
Kernel Classes
The OpenMS kernel contains the data structures that store the actual MS data.
For storing the basic MS data (spectra, chromatograms, and full runs) OpenMS uses
- Peaks (Peak1D and ChromatogramPeak) stored in
- MSSpectrum and MSChromatogram, which in turn can both be stored in an
- MSExperiment
For storing quantified peptides or analytes in single MS runs, OpenMS uses so called feature maps.
The main data structures for quantitative information are
- Features (for quantitative information in MS1 maps)
- MRMFeatures (for quantitative information in XIC traces on MS1 and MS2 level)
- which are both stored in a FeatureMap
To store quantified peptides or analytes over several MS runs, OpenMS uses so called consensus maps.
- ConsensusFeatures are stored in a
- ConsensusMap
To store identified peptides OpenMS has classes
- PeptideHit, which corresponds to a Peptide-Spectrum-Matching stored in a
- PeptideIdentification object (which is associated with a single spectrum)
Directory structure of core library (/src/openms)
| Stored Entity | Class Name |
| Mass Peak (m/z + intensity) | Peak1D |
| Elution Peak (rt + intensity) | ChromatogramPeak |
| Spectrum of Mass Peaks | MSSpectrum |
| Chromatogram of Elution Peaks | MSChromatogram |
| Mass trace for small molecule detection | MassTrace |
| Full MS run, containing both spectra and chromatograms | MSExperiment (alias PeakMap) |
| Feature (isotopic pattern of eluting analyte) | Feature |
| All features detected in an MS Run | FeatureMap |
| Linked / Grouped feature (e.g., same Peptide quantified in several MS runs) | ConsensusFeature |
| All grouped ConsensusFeatures of a multi-run experiment | ConsensusMap |
| Peptide Spectrum Match | PeptideHit |
| Identified Spectrum with one or several PSMs | PeptideIdentification |
| Identified Protein | ProteinHit |
Peaks
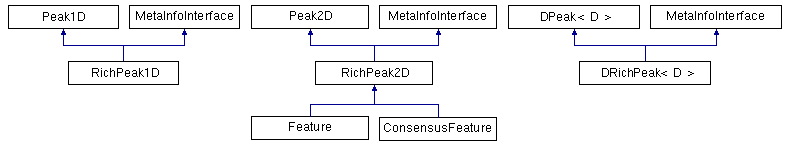
OpenMS provides one-, two- and d-dimensional data points, either with or without metadata attached to them.
Data structure for MS data points
One-dimensional data points: One-dimensional data points (Peak1D) are the most important ones and used throughout OpenMS. The two-dimensional and d-dimensional data points are needed rarely and used for special purposes only. Peak1D provides getter and setter methods to store the mass-to-charge ratio and intensity.
Two-dimensional data points: The two-dimensional data points store mass-to-charge, retention time and intensity. The most prominent example we will later take a closer look at is the Feature class, which stores a two-dimensional position (m/z and RT) and intensity of the eluting peptide or analyte.
The base class of the two-dimensional data points is Peak2D. It provides the same interface as Peak1D and additional getter and setter methods for the retention time. RichPeak2D is derived from Peak2D and adds an interface for metadata. The Feature is derived from RichPeak2D and adds information about the convex hull of the feature, quality and so on.
For information on d-dimensional data points see the appendix.
- Note
- All subsequent code snippets are taken from fully self-contained compilation units in
openms/doc/code_examples, which can be build as executables using the Tutorials_build target.
Spectra
The most important container for raw/profile data and centroided peaks is MSSpectrum. The elements of a MSSpectrum are peaks (Peak1D). In fact it is so common that it has its own typedef PeakSpectrum. MSSpectrum is derived from SpectrumSettings, a container for the metadata of a spectrum (e.g. precursor information). Here, only MS data handling is explained, SpectrumSettings is described in subsection meta data of a spectrum. In the following example (Tutorial_MSSpectrum.cpp) program, a MSSpectrum is filled with peaks, sorted according to mass-to-charge ratio and a selection of peak positions is displayed as One-dimensional data points:
Example: Tutorial_MSSpectrum.cpp
In this example, we create MS1 spectrum at 1 minute and insert peaks with descending mass-to-charge ratios (for educational reasons). We sort the peaks according to ascending mass-to-charge ratio. Finally we print the peak positions of those peaks between 800 and 1000 Thomson. For printing all the peaks in the spectrum, we simply would have used the STL-conform methods begin() and end(). In addition to the iterator access, we can also directly access the peaks via vector indices (e.g. spectrum[0] is the first Peak1D object of the MSSpectrum).
{
for (float mz = 1500.0; mz >= 500; mz -= 100.0)
{
spectrum.push_back(peak);
}
for (
auto it = spectrum.
MZBegin(800.0); it != spectrum.
MZEnd(1000.0); ++it)
{
cout << it->getMZ() << endl;
}
cout << spectrum[1].getMZ() << " " << spectrum[1].getIntensity() << endl;
return 0;
}
int main(int argc, const char **argv)
Definition FLASHDeconvWizard.cpp:73
The representation of a 1D spectrum.
Definition MSSpectrum.h:44
Iterator MZBegin(CoordinateType mz)
Binary search for peak range begin.
void sortByPosition()
Lexicographically sorts the peaks by their position.
Iterator MZEnd(CoordinateType mz)
Binary search for peak range end (returns the past-the-end iterator)
A 1-dimensional raw data point or peak.
Definition Peak1D.h:30
void setMZ(CoordinateType mz)
Mutable access to m/z.
Definition Peak1D.h:95
Main OpenMS namespace.
Definition openswathalgo/include/OpenMS/OPENSWATHALGO/DATAACCESS/ISpectrumAccess.h:19
Chromatograms
The most important container for targeted analysis / XIC data is MSChromatogram. The elements of a MSChromatogram are chromatogram peaks (Peak1D). MSChromatogram is derived from ChromatogramSettings, a container for the metadata of a chromatogram (e.g. containing precursor and product information), similarly to SpectrumSettings. In the following example (Tutorial_MSChromatogram.cpp) program, a MSChromatogram is filled with chromatographic peaks, sorted according to retention time and a selection of peak positions is displayed.
Example: Tutorial_MSChromatogram
Fill MSChromatogram with chromatographic peaks, sorted according to retention time
{
for (float rt = 200.0; rt >= 100; rt -= 1.0)
{
chromatogram.push_back(peak);
}
return 0;
}
A 1-dimensional raw data point or peak for chromatograms.
Definition ChromatogramPeak.h:29
void setRT(CoordinateType rt)
Mutable access to RT.
Definition ChromatogramPeak.h:93
void setIntensity(IntensityType intensity)
Mutable access to the data point intensity (height)
Definition ChromatogramPeak.h:84
const Product & getProduct() const
returns a const reference to the products
const Precursor & getPrecursor() const
returns a const reference to the precursors
void setNativeID(const String &native_id)
sets the native identifier for the spectrum, used by the acquisition software.
The representation of a chromatogram.
Definition MSChromatogram.h:30
void setMZ(double mz)
sets the target m/z
Since much of the functionality is shared between MSChromatogram and MSSpectrum, further examples can be gathered from the MSSpectrum subsection.
Precursor
The precursor data stored along with MS/MS spectra contains invaluable information for MS/MS analysis (e.g, m/z, charge, activation mode, collision energy). This information is stored in Precursor objects that can be retrieved from each spectrum. For a complete list of functions please see the Precursor class documentation.
Example: Tutorial_Precursor
Retrieve precursor information
#include <OpenMS/openms_data_path.h>
#include <iostream>
int main(
int argc,
const char** argv)
{
auto file_mzML = OPENMS_DOC_PATH +
String(
"/code_examples/data/Tutorial_GaussFilter.mzML");
for (
auto s_it = spectra.
begin(); s_it != spectra.
end(); ++s_it)
{
if (s_it->getMSLevel() != 2) continue;
if (precursors.empty())
throw Exception::InvalidSize(__FILE__, __LINE__, OPENMS_PRETTY_FUNCTION, precursors.size(),
"precursors vector must not be empty");
double precursor_mz = precursors[0].getMZ();
float precursor_int = precursors[0].getIntensity();
double precursor_rt = precursor_spectrum->getRT();
std::cout << " precursor m/z: " << precursor_mz
<< " intensity: " << precursor_int
<< " retention time (sec.): " << precursor_rt
<< std::endl;
}
return 0;
}
Invalid UInt exception.
Definition Exception.h:276
Facilitates file handling by file type recognition.
Definition FileHandler.h:46
void loadExperiment(const String &filename, PeakMap &exp, const std::vector< FileTypes::Type > allowed_types=std::vector< FileTypes::Type >(), ProgressLogger::LogType log=ProgressLogger::NONE, const bool rewrite_source_file=false, const bool compute_hash=false)
Loads a file into an MSExperiment.
In-Memory representation of a mass spectrometry run.
Definition MSExperiment.h:49
Iterator begin() noexcept
ConstIterator getPrecursorSpectrum(ConstIterator iterator) const
Returns the precursor spectrum of the scan pointed to by iterator.
std::vector< SpectrumType >::const_iterator ConstIterator
Non-mutable iterator.
Definition MSExperiment.h:86
const std::vector< Precursor > & getPrecursors() const
returns a const reference to the precursors
A more convenient string class.
Definition String.h:34
MRMTransitionGroup
The targeted analysis of SRM or DIA (SWATH-MS) type of data requires a set of targeted assays as well as raw data chromatograms. The MRMTransitionGroup class allows users to map these two types of information and store them together with identified features conveniently in a single object.
Create an empty MRMTransitionGroup with two dummy transitions
using TrGroup = MRMTransitionGroup<MSChromatogram, ReactionMonitoringTransition>;
TrGroup createTransitionGroup()
{
TrGroup tr_group;
tr_group.addChromatogram(MSChromatogram(), “transition1”);
tr_group.addTransition(ReactionMonitoringTransition(), “transition1”);
tr_group.addChromatogram(MSChromatogram(), “transition2”);
tr_group.addTransition(ReactionMonitoringTransition(), “transition2”);
tr_group.setTransitionGroupID(“tr_peptideA”);
return tr_group;
}
Note how the identifiers of the chromatograms and the assay information (ReactionMonitoringTransition) are matched so that downstream algorithms can utilize the meta-information stored in the assays for data analysis.
Maps
Although raw data maps, peak maps and feature maps are conceptually very similar they are stored in different data types. For raw data and peak maps, the default container is MSExperiment, which is an array of MSSpectrum instances. In contrast to raw data and peak maps, feature maps are not a collection of one-dimensional spectra, but an array of two-dimensional feature instances. The main data structure for feature maps is called FeatureMap.
Although MSExperiment and FeatureMap differ in the data they store, they also have things in common. Both store metadata that is valid for the whole map, i.e. sample description and instrument description. This data is stored in the common base class ExperimentalSettings.
MSExperiment
MSExperiment contains ExperimentalSettings (metadata of the MS run) and a vector<MSSpectrum>. The one-dimensional spectrum MSSpectrum is derived from SpectrumSettings (metadata of a spectrum).
Example: Tutorial_MSExperiment.cpp
The following example creates a MSExperiment containing four MSSpectrum instances. We then iterate over RT range (2,3) and m/z range (603,802) and print the peak positions using an AreaIterator. Then we show how we iterate over all spectra and peaks. In the commented out part, we show how to load/store all spectra and associated metadata from/to an mzML file.
#include <iostream>
{
for (
Size i = 0; i < 4; ++i)
{
for (float mz = 500.0; mz <= 900; mz += 100.0)
{
spectrum.push_back(peak);
}
}
for (
auto it = exp.
areaBegin(2.0, 3.0, 603.0, 802.0); it != exp.
areaEnd(); ++it)
{
cout << it.getRT() << " - " << it->getMZ() << endl;
}
for (
auto s_it = exp.
begin(); s_it != exp.
end(); ++s_it)
{
for (auto p_it = s_it->begin(); p_it != s_it->end(); ++p_it)
{
cout << s_it->getRT() << " - " << p_it->getMZ() << " " << p_it->getIntensity() << endl;
}
}
std::cout << "Data ranges:\n";
std::cout <<
"\nGet maximum intensity on its own: " << exp.
spectrumRanges().getMaxIntensity() <<
'\n';
std::cout <<
"Get minimum RT on its own: " << exp.
spectrumRanges().getMinRT() <<
'\n';
std::cout <<
"Get maximum RT on its own: " << exp.
spectrumRanges().getMaxRT() <<
'\n';
std::cout <<
"Get minimum m/z on its own: " << exp.
spectrumRanges().getMinMZ() <<
'\n';
std::cout <<
"Get maximum m/z on its own: " << exp.
spectrumRanges().getMaxMZ() <<
'\n';
{
std::cout <<
"Get minimum IM on its own: " << exp.
spectrumRanges().getMinMobility() <<
'\n';
std::cout <<
"Get maximum IM on its own: " << exp.
spectrumRanges().getMaxMobility() <<
'\n';
}
auto tmp_filename = File::getTemporaryFile();
return 0;
}
void storeExperiment(const String &filename, const PeakMap &exp, const std::vector< FileTypes::Type > allowed_types={}, ProgressLogger::LogType log=ProgressLogger::NONE)
Stores an MSExperiment to a file.
PeakFileOptions & getOptions()
Mutable access to the options for loading/storing.
void addSpectrum(const MSSpectrum &spectrum)
adds a spectrum to the list
AreaIterator areaBegin(CoordinateType min_rt, CoordinateType max_rt, CoordinateType min_mz, CoordinateType max_mz, UInt ms_level=1)
Returns an area iterator for area.
void updateRanges()
Updates the m/z, intensity, mobility, and retention time ranges of all spectra and chromatograms.
const SpectrumRangeManagerType & spectrumRanges() const
Returns a const reference to the spectrum range manager.
Definition MSExperiment.h:1237
AreaIterator areaEnd()
Returns an invalid area iterator marking the end of an area.
void setMSLevel(UInt ms_level)
Sets the MS level.
void setRT(double rt)
Sets the absolute retention time (in seconds)
void setMSLevels(const std::vector< Int > &levels)
sets the desired MS levels for peaks to load
void printRange(std::ostream &out) const
print each dimension (base classes) to a stream
Definition RangeManager.h:851
size_t Size
Size type e.g. used as variable which can hold result of size()
Definition Types.h:97
FeatureMap
FeatureMap, the container for features, is simply a vector<Feature>. Additionally, it is derived from ExperimentalSettings, to store the meta information. All peak and feature containers (MSSpectrum, MSExperiment, FeatureMap) are also derived from RangeManager. This class facilitates the handling of MS data ranges. It allows to calculate and store both the position range and the intensity range of the container.
Example: Tutorial_FeatureMap.cpp
The following examples creates a FeatureMap containing two Feature instances. Then we iterate over all features and output the retention time and m/z. We then show, how to use the underlying range manager to retrieve FeatureMap boundaries in rt, m/z, and intensity.
#include <iostream>
{
for (auto& f : map)
{
cout << f.getRT() << " - " << f.getMZ() << endl;
}
cout << "Int: " << map.getMinIntensity() << " - " << map.getMaxIntensity() << endl;
cout << "RT: " << map.getMinRT() << " - " << map.getMaxRT() << endl;
cout << "m/z: " << map.getMinMZ() << " - " << map.getMaxMZ() << endl;
}
void push_back(const VectorElement &f)
Definition ExposedVector.h:168
A container for features.
Definition FeatureMap.h:82
void updateRanges() override
An LC-MS feature.
Definition Feature.h:46
void setMZ(CoordinateType coordinate)
Mutable access to the m/z coordinate (index 1)
Definition Peak2D.h:179
void setRT(CoordinateType coordinate)
Mutable access to the RT coordinate (index 0)
Definition Peak2D.h:191
File Formats
| mzML | The HUPO-PSI standard format for mass spectrometry data |
| mzIdentML | The HUPO-PSI standard format for identification results data from any search engines |
| mzTAB | The HUPO-PSI standard format for reporting MS-based proteomics and metabolomics results |
| traML | The HUPO-PSI standard format for exchange and transmission lists for selected reaction monitoring (SRM) experiments |
| featureXML | The OpenMS format for quantitation results |
| consensusXML | The OpenMS format for grouping features in one map or across several maps |
| idXML | The OpenMS format for identification results |
| trafoXML | The OpenMS format for storing of transformations |
| OpenSWATH | |
For further information of the HUPO Proteomics Standards Initiative please visit: http://www.psidev.info/
Identifications
Identifications of proteins, peptides, and the mapping between peptides and proteins (or groups of proteins) are stored in dedicated data structures. These data structures are typically stored to disc as idXML or mzIdentML file. The highest-level structure is ProteinIdentification. It stores all identified proteins of an identification run as ProteinHit objects + additional metadata (search parameters, etc.). Each ProteinHit contains the actual protein accession, an associated score, and (optionally) the protein sequence. A ProteinIdentification object stores the data corresponding to a single identified spectrum or feature. It has members for the retention time, m/z, and a vector of PeptideHits. Each PeptideHit stores the information of a specific peptide-to-spectrum match (e.g., the score and the peptide sequence). Each PeptideHit also contains a vector of PeptideEvidence objects which store the reference to one (or in the case the peptide maps to multiple proteins multiple) Proteins and the position therein.
Example: Tutorial_IdentificationClasses.cpp
Create all identification data needed to store an idXML file
#include <iostream>
{
vector<ProteinHit> protein_hits;
protein_hit.
setSequence(
"PEPTIDEPEPTIDEPEPTIDEPEPTIDER");
protein_hits.push_back(protein_hit);
search_parameters.
db =
"database";
vector<ProteinIdentification> protein_ids;
protein_ids.push_back(protein_id);
for (const auto& prot : protein_ids)
{
for (const auto& hit : prot.getHits())
{
cout << "Protein hit accession: " << hit.getAccession() << '\n';
cout << "Protein hit sequence: " << hit.getSequence() << '\n';
cout << "Protein hit score: " << hit.getScore() << '\n';
}
}
peptide_id.
setRT(1243.56);
vector<PeptideHit> peptide_hits;
peptide_hit.
setSequence(AASequence::fromString(
"DLQM(Oxidation)TQSPSSLSVSVGDR"));
peptide_hits.push_back(peptide_hit);
peptide_hit.
setSequence(AASequence::fromString(
"QLDM(Oxidation)TQSPSSLSVSVGDR"));
peptide_hits.push_back(peptide_hit);
for (const auto& peptide_id : peptide_ids)
{
cout <<
"Peptide ID m/z: " << peptide_id.
getMZ() <<
'\n';
cout <<
"Peptide ID rt: " << peptide_id.
getRT() <<
'\n';
cout <<
"Peptide ID score type: " << peptide_id.
getScoreType() <<
'\n';
for (const auto& scored_hit : peptide_id.getHits())
{
cout << " - Peptide hit rank: " << scored_hit.getRank() << '\n';
cout << " - Peptide hit sequence: " << scored_hit.getSequence().toString() << '\n';
cout << " - Peptide hit score: " << scored_hit.getScore() << '\n';
}
}
}
DateTime Class.
Definition DateTime.h:35
void getDate(UInt &month, UInt &day, UInt &year) const
Fills the arguments with the date.
Represents a single spectrum match (candidate) for a specific tandem mass spectrum (MS/MS).
Definition PeptideHit.h:52
void setSequence(const AASequence &sequence)
sets the peptide sequence
void setRank(UInt newrank)
sets the PSM rank (0 = top hit)
void setCharge(Int charge)
sets the charge of the peptide
void setScore(double score)
sets the PSM score
Container for peptide identifications from multiple spectra.
Definition PeptideIdentificationList.h:66
Represents the set of candidates (SpectrumMatches) identified for a single precursor spectrum.
Definition PeptideIdentification.h:66
void setIdentifier(const String &id)
sets the identifier which links this PI to its corresponding ProteinIdentification
double getRT() const
returns the RT of the MS2 spectrum where the identification occurred
const String & getScoreType() const
returns the peptide score type
void setMZ(double mz)
sets the MZ of the MS2 spectrum
void setScoreType(const String &type)
sets the peptide score type
void setHigherScoreBetter(bool value)
sets the peptide score orientation
void setHits(const std::vector< PeptideHit > &hits)
Sets the peptide hits.
void setRT(double rt)
sets the RT of the MS2 spectrum where the identification occurred
double getMZ() const
returns the MZ of the MS2 spectrum
Representation of a protein hit.
Definition ProteinHit.h:35
void setSequence(const String &sequence)
sets the protein sequence
void setScore(const double score)
sets the score of the protein hit
void setAccession(const String &accession)
sets the accession of the protein
Representation of a protein identification run.
Definition ProteinIdentification.h:54
void setIdentifier(const String &id)
Sets the identifier.
void setSearchEngine(const String &search_engine)
Sets the search engine type.
void setSearchEngineVersion(const String &search_engine_version)
Sets the search engine version.
void setHits(const std::vector< ProteinHit > &hits)
Sets the protein hits.
void setScoreType(const String &type)
Sets the protein score type.
void setDateTime(const DateTime &date)
Sets the date of the protein identification run.
void setSearchParameters(const SearchParameters &search_parameters)
Sets the search parameters.
Search parameters of the DB search.
Definition ProteinIdentification.h:217
String charges
The allowed charges for the search.
Definition ProteinIdentification.h:221
String db
The used database.
Definition ProteinIdentification.h:218
Chemistry
Element, ElementDB, EmpiricalFormula
An Element object is the representation of an element. It can store the name, symbol and mass (average/mono) and natural abundances of isotopes. Elements are retrieved from the ElementDB singleton. The EmpiricalFormula object can be used to represent the empirical formula of a compound as well as to extract its natural isotope abundance and weight.
Example: Tutorial_Element.cpp
Work with Element object
#include <iostream>
#include <iomanip>
{
const ElementDB& db = *ElementDB::getInstance();
if (db.
hasElement(
"foo")) { std::cout <<
"worth a try..."; }
const auto all_elements_name = db.
getNames();
const auto all_elements_symbols = db.
getSymbols();
std::cout << "We currently know of: " << all_elements_name.size() << " elements (incl. isotopes)\n"
<< " with: " << all_elements_AN.size() << " different atomic numbers (linking to the monoisotopic isotope)\n"
<< " and: " << all_elements_symbols.size() << " different symbols\n\n";
std::cout << "\nLet's find all hydrogen isotopes:\n";
for (const auto e : all_elements_name)
{
if (e.second->getAtomicNumber() == 1)
{
std::cout << " --> " << std::setw(30) << e.first
<< " Symbol: " << std::setw(5) << e.second->getSymbol()
<< " AN: " << std::setw(3) << e.second->getAtomicNumber()
<< " mono-weight: " << std::setw(14)<< e.second->getMonoWeight() << "\n";
}
}
std::cout << "\nLets print all monoisotopic elements:\n";
for (const auto e : all_elements_AN)
{
std::cout << std::setw(30) << e.first
<< " Symbol: " << std::setw(5) << e.second->getSymbol()
<< " AN: " << std::setw(3) << e.second->getAtomicNumber()
<< " mono-weight: " << std::setw(14)<< e.second->getMonoWeight() << "\n";
}
}
Singleton that stores elements and isotopes.
Definition ElementDB.h:45
const std::unordered_map< std::string, const Element * > & getSymbols() const
returns a hashmap that contains symbols mapped to pointers to the elements
const Element * getElement(const std::string &name) const
bool hasElement(const std::string &name) const
returns true if the db contains an element with the given name
const std::unordered_map< unsigned int, const Element * > & getAtomicNumbers() const
returns a hashmap that contains atomic numbers mapped to pointers of the elements
const std::unordered_map< std::string, const Element * > & getNames() const
returns a hashmap that contains names mapped to pointers to the elements
Representation of an element.
Definition Element.h:34
const std::string & getSymbol() const
returns symbol of the element
double getMonoWeight() const
returns the mono isotopic weight of the element
const std::string & getName() const
returns the name of the element
double getAverageWeight() const
returns the average weight of the element
int Int
Signed integer type.
Definition Types.h:72
Example: Tutorial_EmpiricalFormula.cpp
Extract isotope distribution and monoisotopic weight of an EmpiricalFormula object
#include <iostream>
{
const Element * carbon = ElementDB::getInstance()->getElement(
"Carbon");
cout << "Formula: " << sum
std::cout << "\n\nCoarse isotope distribution of " << sum << ": \n";
for (const auto& it : iso_dist)
{
cout << "m/z: " << it.getMZ() << " abundance: " << it.getIntensity() << endl;
}
}
Isotope pattern generator for coarse isotope distributions.
Definition CoarseIsotopePatternGenerator.h:79
Definition IsotopeDistribution.h:40
AASequence - Representing a Peptide (amino acid sequence)
An AASequence object stores a (potentially chemically modified) peptide. It can conveniently be constructed from the amino acid sequence (e.g., a string or a string literal “DEFIANGR”). Modifications may be encoded using the unimod name. Once constructed, many convenient functions are available to calculate peptide or ion properties.
Example: Tutorial_AASequence.cpp
Compute and output basic AASequence properties
#include <iostream>
{
AASequence peptide2 = AASequence::fromString(
"PEPTIDER");
cout << peptide1.
toString() <<
" " << prefix <<
" " << suffix << endl;
AASequence peptide_meth_ox = AASequence::fromString(
"PEPTIDESEKUEM(Oxidation)CER");
cout << "Monoisotopic mass of the uncharged, full peptide: " << peptide_mass_mono << endl;
cout << "Average mass of the uncharged, full peptide: " << peptide_mass_avg << endl;
cout << "Mass of the doubly positively charged b3-ion: " << ion_mass_b3_2plus << endl;
cout <<
"Mass-to-charge of the doubly positively charged b3-ion: " << peptide_meth_ox.
getPrefix(3).
getMZ(2, Residue::BIon) << endl;
cout <<
"Mass-to-charge of the doubly positively charged peptide: " << peptide_meth_ox.
getMZ(2) << endl;
std::map<String, Size> aa_freq;
cout <<
"Number of Proline (P) residues in '" << peptide_meth_ox.
toString() <<
"' is " << aa_freq[
'P'] << endl;
return 0;
}
Representation of a peptide/protein sequence.
Definition AASequence.h:88
double getMZ(Int charge, Residue::ResidueType type=Residue::Full) const
String toString() const
returns the peptide as string with modifications embedded in brackets
double getAverageWeight(Residue::ResidueType type=Residue::Full, Int charge=0) const
returns the average weight of the peptide
AASequence getPrefix(Size index) const
returns a peptide sequence of the first index residues
double getMonoWeight(Residue::ResidueType type=Residue::Full, Int charge=0) const
void getAAFrequencies(std::map< String, Size > &frequency_table) const
compute frequency table of amino acids
String toUnmodifiedString() const
returns the peptide as string without any modifications or (e.g., "PEPTIDER")
AASequence getSuffix(Size index) const
returns a peptide sequence of the last index residues
Internally, an AASequence object is composed of Residues.
Residue and ResidueDB - Dealing with residues / amino acids
Residues are the building blocks of AASequence objects. They store physico-chemical properties of amino acids. ResidueDB provides access to different residue sets (e.g. all natural AAs).
Example: Tutorial_Residue.cpp
Compute and output basic Residue properties
#include <iostream>
{
ResidueDB const * res_db = ResidueDB::getInstance();
AASequence aas = AASequence::fromString(
"DEFIANGER");
cout << aas[3].getName() << " "
return 0;
}
EmpiricalFormula getFormula(Residue::ResidueType type=Residue::Full, Int charge=0) const
returns the formula of the peptide
OpenMS stores a central database of all residues in the ResidueDB. All (unmodified) residues are adde...
Definition ResidueDB.h:32
const Residue * getResidue(const String &name) const
returns a pointer to the residue with name, 3 letter code or 1 letter code name
Representation of an amino acid residue.
Definition Residue.h:40
const String & getOneLetterCode() const
returns the name as one letter code (String of size 1)
EmpiricalFormula getFormula(ResidueType res_type=Full) const
returns the empirical formula of the residue
const String & getThreeLetterCode() const
returns the name of the residue as three letter code (String of size 3)
const String & getName() const
returns the name of the residue
double getAverageWeight(ResidueType res_type=Full) const
returns average weight of the residue
double getMonoWeight(ResidueType res_type=Full) const
returns monoisotopic weight of the residue
ResidueModification, ModificationsDB
If a residue is modified (e.g. phosphorylation of an amino acid) it can be stored in the ResidueModification class. The ResidueModification class stores information about chemical modifications of residues. Each ResidueModification has an ID, the residue that can be modified with this modification and the difference in mass between the unmodified and the modified residue, among other information. The Residue class allows to set one modification per residue and the mass difference of the modification is accounted for in the mass of the residue. The class ModificationsDB is a database of ResidueModifications. These are mostly initialized from the file “/share/CHEMISTRY/unimod.xml” containing a slightly modified version of the UniMod database of modifications. ModificationsDB has functions to search for modifications by name or mass.
Example: Tutorial_ResidueModification.cpp
Set a ResidueModification on a Residue
#include <iostream>
{
AASequence aas = AASequence::fromString(
"DECIANGER");
cout << aas[2].getName() << " "
<< aas[2].getModificationName() << " "
const ResidueModification* mod = ModificationsDB::getInstance()->getModification(
"Carbamidomethyl (C)");
cout << aas[2].getName() << " "
<< aas[2].getModificationName() << " "
return 0;
}
void setModification(Size index, const String &modification)
Representation of a modification on an amino acid residue.
Definition ResidueModification.h:55
const String & getFullId() const
returns the full identifier of the mod (Unimod accession + origin, if available)
double getDiffMonoMass() const
returns the diff monoisotopic mass, or 0.0 if not set
char getOrigin() const
Returns the origin (i.e. modified amino acid)
double getMonoMass() const
return the monoisotopic mass, or 0.0 if not set
TheoreticalSpectrumGenerator
The TheoreticalSpectrumGenerator generates ion ladders from AASequences.
Example: Tutorial_TheoreticalSpectrumGenerator.cpp
Generate theoretical spectra
#include <iostream>
{
tsg_settings.
setValue(
"add_a_ions",
"true");
tsg_settings.
setValue(
"add_metainfo",
"true");
AASequence peptide = AASequence::fromString(
"DEFIANGER");
cout << "Mass of second peak: " << theoretical_spectrum[1].getMZ()
<< " | Ion type of second peak: " << ion_types[1] << endl;
cout << "Mass of tenth peak: " << theoretical_spectrum[9].getMZ()
<< " | Ion type of tenth peak: " << ion_types[9] << endl;
return 0;
}
String data array class.
Definition DataArrays.h:125
const Param & getParameters() const
Non-mutable access to the parameters.
void setParameters(const Param ¶m)
Sets the parameters.
const StringDataArrays & getStringDataArrays() const
Returns a const reference to the string meta data arrays.
Management and storage of parameters / INI files.
Definition Param.h:46
void setValue(const std::string &key, const ParamValue &value, const std::string &description="", const std::vector< std::string > &tags=std::vector< std::string >())
Sets a value.
Generates theoretical spectra for peptides with various options.
Definition TheoreticalSpectrumGenerator.h:45
virtual void getSpectrum(PeakSpectrum &spec, const AASequence &peptide, Int min_charge, Int max_charge, Int precursor_charge=0) const
DigestionEnzymeProtein, ProteaseDB and ProteaseDigestion
OpenMS provides the most common digestion enzymes (DigestionEnzymeProtein) used in MS. They are stored in the ProteaseDB singleton and loaded from “/share/CHEMISTRY/Enzymes.xml”.
Example: Tutorial_Enzyme.cpp
Digest amino acid sequence
#include <vector>
#include <iostream>
{
cout << protease.
peptideCount(AASequence::fromString(
"ACKPDE")) <<
" " << protease.
peptideCount(AASequence::fromString(
"ACRPDEKA"))
<< endl;
vector<AASequence> products;
auto aa_seq = AASequence::fromString("ARCDRE.(Amidated)");
protease.
digest(aa_seq, products);
std::cout << "digesting " << aa_seq.toString() << " into:\n";
{
cout << "--> " << p.toString() << "\n";
}
cout << endl;
protease.
digest(aa_seq, products);
std::cout << "digesting " << aa_seq.toString() << " with 10 MCs into:\n";
{
cout << "--> " << p.toString() << "\n";
}
cout << endl;
cout <<
"Is '" << peptide.
prefix(4) <<
"' a valid digestion product of '" << peptide <<
"'? "
}
void setMissedCleavages(Size missed_cleavages)
Sets the number of missed cleavages for the digestion (default is 0). This setting is ignored when lo...
Class for the enzymatic digestion of proteins represented as AASequence or String.
Definition ProteaseDigestion.h:32
bool isValidProduct(const String &protein, int pep_pos, int pep_length, bool ignore_missed_cleavages=true, bool allow_nterm_protein_cleavage=false, bool allow_random_asp_pro_cleavage=false) const
Variant of EnzymaticDigestion::isValidProduct() with support for n-term protein cleavage and random D...
Size peptideCount(const AASequence &protein)
Returns the number of peptides a digestion of protein would yield under the current enzyme and missed...
void setEnzyme(const String &name)
Sets the enzyme for the digestion (by name)
Size digest(const AASequence &protein, std::vector< AASequence > &output, Size min_length=1, Size max_length=0) const
Performs the enzymatic digestion of a protein represented as AASequence. Digestion logic is implement...
String prefix(SizeType length) const
returns the prefix of length length
Tool development
TOPP-Tool
TOPP (The OpenMS Pipeline) tools are small command line applications built using the OpenMS library. They act as building blocks for complex analysis workflows and may perform e.g. simple signal processing tasks like filtering, up to more complex tasks like protein inference and quantitation over several MS runs. Common to all TOPP tools is a command line interface allowing automatic integration into workflow engines like KNIME. They are the preferred way to integrate novel methods as application into OpenMS. When we first create a novel TOPP tool it is considered unstable. To set it apart from the stable and well tested tools it gets first created as TOPP Util (note: the name “util” has historic reasons and may be changed to unstable tools in the future). If it is well tested it will be promoted to a stable Tool in future OpenMS versions.
Imagine that you want to create a new tool that allows filtering of sequence databases. What you usually would first do is check if such or similar functionality has already been implemented in any of the >150 TOPP tools. If you are unsure which one to use, just ask on the mailing list, the gitter chat or contact one of the developers directly. The following subsection demonstrates how the original “DatabaseFilter” tool was created from scratch an integrated into OpenMS. Basically any tool you want to integrate needs to follow the steps outlined below.
But let’s first get started by defining what our tool should actually do: The DatabaseFilter tool should provide functionality to reduce a fasta database by filtering its entries based on different criteria. A simple criterion could be the length of a protein. To make the task a bit more interesting and to show other parts of the OpenMS library, we will start with a bit more complex filtering step that keeps all entries from the fasta database that have been identified in a peptide search (e.g., using X!Tandem, Mascot or MSGF+). This functionality might come in handy if the size of large databases needs to be reduced to a manageable size. In addition, we want the user to be able to choose between keeping and removing matching protein id.
Create and register a minimal tool in OpenMS
- Create an empty file src/utils/DatabaseFilter.cpp
- Add the scaffold code for a minimal TOPP tool. Text in bold will later be adapted to our DatabaseFilter tool.
Example: Tutorial_Template.cpp
Template for OpenMS tool development
class TOPPNewTool :
{
public:
TOPPNewTool() :
TOPPBase(
"NewTool",
"Template for Tool creation", false)
{
}
protected:
void registerOptionsAndFlags_()
{
}
ExitCodes main_(int, const char **)
{
return ExitCodes::EXECUTION_OK;
}
};
int main(
int argc,
const char ** argv)
{
TOPPNewTool tool;
return tool.main(argc, argv);
}
Base class for TOPP applications.
Definition TOPPBase.h:122
- Now add a line with DatabaseFilter.cpp to src/utils/executables.cmake. This registers the novel tool in the OpenMS build system.
- Then add the tool to getUtilList() in src/openms/source/APPLICATIONS/ToolHandler.cpp This creates a manual (doxygen) page with the information –help output of the tool (using TOPPDocumenter). This page must be included at the end of the doxygen documentation of your tool (see other tools for an example).
- Add yourself as Maintainer/Author
- Write the basic documentation (doxygen docu). You probably need to refine it later but you can already insert the correct Toolname etc..
Define tool parameters
Define tool parameters Each TOPP tool defines a set of parameters that will be available from the command line, KNIME, and other workflow systems. This is done in the void registerOptionsAndFlags_() method. In our case we want to read a protein database (fasta format), a file containing identification data (idXML format), and an option to switch between keeping (whitelisting) and removing (blacklisting) entries based on the filter result. This is our input. The reduced database forms the output and should be written to a protein database in fasta format. This is easily done by adding following lines to:
Example: Tutorial_TOPP.cpp
Registration of tool parameters
void registerOptionsAndFlags_() override
{
registerInputFile_("in", "<file>", "", "Input FASTA file, containing a protein database.");
setValidFormats_("in", {"fasta"});
registerInputFile_("id", "<file>", "", "Input file containing identified peptides and proteins.");
setValidFormats_("id", {"idXML", "mzid"});
registerStringOption_("method", "<choice>", "whitelist", "Switch between white-/blacklisting of protein IDs", false);
setValidStrings_("method", {"whitelist", "blacklist"});
registerOutputFile_("out", "<file>", "", "Output FASTA file where the reduced database will be written to.");
setValidFormats_("out", {"fasta"});
}
Functions, classes and references can be checked in the OpenMS / TOPP documentation (http://www.openms.de/current_doxygen/html/)
Read tool parameters
After a tool is executed, the registered parameters are available in the main_ function of the TOPP tool and can be read using the getStringOption_ method. Special methods for integers, lists and floating point parameters exist and are in the TOPPBase documentation but are not needed for this example.
Example: Tutorial_TOPP.cpp
String in(getStringOption_(
"in"));
String ids(getStringOption_(
"id"));
String method(getStringOption_(
"method"));
bool whitelist = (method == "whitelist");
String out(getStringOption_(
"out"));
Read Input Files
First the different file formats and data structures for peptide identifications have to be included at the top of the file.
Example: Tutorial_TOPP.cpp
Add essential includes
Read the input files
vector<FASTAFile::FASTAEntry> db;
This class serves for reading in and writing FASTA files If the protein/gene sequence contains unusua...
Definition FASTAFile.h:35
void load(const String &filename, std::vector< FASTAEntry > &data) const
loads a FASTA file given by 'filename' and stores the information in 'data' This uses more RAM than r...
Note: both peptide_identifications and protein_identifications contain protein accessions. The difference between them is that protein_identifications only contain the inferred set of protein accessions while peptide_identifications contains all protein accessions the peptides map to. We consider only the larger set of protein accessions stored in the peptide identifications. In principle, it would be easy to add another parameter that adds a filter for the inferred accessions stored in protein_identifications.
Add the tool functionality
First, the accessions are extracted from the IdXML file. Here knowledge of the data structure is needed to extract the protein accessions. The class PeptideIdentification stores general information about a single identified spectrum (e.g., retention time, precursor mass-to-charge). A vector of PeptideHits is stored in each PeptideIdentification object and represent the potentially multiple PSMs of a single spectrum. They can be returned by calling .getHits(). Each peptide sequence stored in a PeptideHit may map to one or multiple proteins. This peptide to protein mapping information is stored in a vector of PeptideEvidence accessible by .getPeptideEvidences(). From each of these evidences we can extract the protein accession with .getProteinAccession().
To store all proteins accessions in the set id_accessions, we write:
Example: Tutorial_TOPP.cpp
Store protein accessions
void filterByProteinAccessions_(const vector<FASTAFile::FASTAEntry>& db,
bool whitelist,
vector<FASTAFile::FASTAEntry>& db_new)
{
set<String> id_accessions;
for (const auto& pep_id : peptide_identifications)
{
for (const auto& hit : pep_id.getHits())
{
for (const auto& ev : hit.getPeptideEvidences())
{
const String& id_accession = ev.getProteinAccession();
id_accessions.insert(id_accession);
}
}
}
Now that we assembled the set of all protein accessions we are ready to compare them to the fasta_accessions. If they are similar and the method whitelist or they are different and the method blacklist was chosen, the fasta entries are copied to the new fasta database.
Example: Tutorial_TOPP.cpp
Add method functionality
for (const auto entry : db)
{
const String& fasta_accession = entry.identifier;
const bool found = id_accessions.find(fasta_accession) != id_accessions.end();
if ((found && whitelist) || (! found && ! whitelist))
{
db_new.push_back(entry);
}
}
Write Output Files
Example: Tutorial_TOPP.cpp
Write the output
void store(const String &filename, const std::vector< FASTAEntry > &data) const
stores the data given by 'data' at the file 'filename'
Adding TOPP tests
Testing your tools is essential and all official TOPP tools need to be tested. Our particular test requires a .fasta and a compatible .idXML file. We add those to /src/tests/topp/. Furthermore, the actual calls to the TOPP tool using our test data, are added to CMakeLists.txt in the same folder, e.g.:
# DatabaseFilter test:
add_test("TOPP_DatabaseFilter_1" ${TOPP_BIN_PATH}/DatabaseFilter -test -in ${DATA_DIR_TOPP}/DatabaseFilter_1.fasta -accession ${DATA_DIR_TOPP}/DatabaseFilter_1.idXML -out DatabaseFilter_1_out.fasta.tmp)
add_test("TOPP_DatabaseFilter_1_out" ${DIFF} -in1 DatabaseFilter_1_out.fasta.tmp -in2 ${DATA_DIR_TOPP}/DatabaseFilter_1_out.fasta )
set_tests_properties("TOPP_DatabaseFilter_1_out" PROPERTIES DEPENDS "TOPP_DatabaseFilter_1")
add_test(
"TOPP_DatabaseFilter_2" ${TOPP_BIN_PATH}/DatabaseFilter -
test -in ${DATA_DIR_TOPP}/DatabaseFilter_1.fasta -accession ${DATA_DIR_TOPP}/DatabaseFilter_1.idXML -out DatabaseFilter_2_out.fasta.tmp -method blacklist)
add_test("TOPP_DatabaseFilter_2_out" ${DIFF} -in1 DatabaseFilter_2_out.fasta.tmp -in2 ${DATA_DIR_TOPP}/DatabaseFilter_2_out.fasta )
set_tests_properties("TOPP_DatabaseFilter_2_out" PROPERTIES DEPENDS "TOPP_DatabaseFilter_2")
bool test
Status of the current subsection.
These tests run the program with the given parameters and then call a diff tool to compare the generated output to the expected output.
Finish documentation
We add it to the UTILS docu page (in doc/doxygen/public/UTILS.doxygen). Later (when we have a working application) we will write an application test (this is optional but recommended for Utils. For Tools it is mandatory). See TOPP tools above and add the test to the bottom of src/tests/topp/CMakeLists.txt.
Polish your code
This is how a util should look after code polishing: Here, the support for different formats was extended (idXML and MZIdentML). Since different filter criteria may be introduced in the future, the structure was slightly changed with a function for the filtering by ID (filterByProteinIDs_) - in order to allow higher flexibility when adding new a functionality later on.
Example: Tutorial_TOPP.cpp
Polish your code - add additional functionality
class TOPPDatabaseFilter :
public TOPPBase
{
public:
TOPPDatabaseFilter():
TOPPBase(
"DatabaseFilter",
"Filters a protein database (FASTA format) based on identified proteins", false)
{
}
protected:
void registerOptionsAndFlags_() override
{
registerInputFile_("in", "<file>", "", "Input FASTA file, containing a protein database.");
setValidFormats_("in", {"fasta"});
registerInputFile_("id", "<file>", "", "Input file containing identified peptides and proteins.");
setValidFormats_("id", {"idXML", "mzid"});
registerStringOption_("method", "<choice>", "whitelist", "Switch between white-/blacklisting of protein IDs", false);
setValidStrings_("method", {"whitelist", "blacklist"});
registerOutputFile_("out", "<file>", "", "Output FASTA file where the reduced database will be written to.");
setValidFormats_("out", {"fasta"});
}
void filterByProteinAccessions_(const vector<FASTAFile::FASTAEntry>& db,
bool whitelist,
vector<FASTAFile::FASTAEntry>& db_new)
{
set<String> id_accessions;
for (const auto& pep_id : peptide_identifications)
{
for (const auto& hit : pep_id.getHits())
{
for (const auto& ev : hit.getPeptideEvidences())
{
const String& id_accession = ev.getProteinAccession();
id_accessions.insert(id_accession);
}
}
}
OPENMS_LOG_INFO <<
"Number of Protein IDs: " << id_accessions.size() << endl;
for (const auto entry : db)
{
const String& fasta_accession = entry.identifier;
const bool found = id_accessions.find(fasta_accession) != id_accessions.end();
if ((found && whitelist) || (! found && ! whitelist))
{
db_new.push_back(entry);
}
}
}
ExitCodes main_(int, const char**) override
{
String in(getStringOption_(
"in"));
String ids(getStringOption_(
"id"));
String method(getStringOption_(
"method"));
bool whitelist = (method == "whitelist");
String out(getStringOption_(
"out"));
vector<FASTAFile::FASTAEntry> db;
vector<ProteinIdentification> protein_identifications;
vector<FASTAFile::FASTAEntry> db_new;
filterByProteinAccessions_(db, peptide_identifications, whitelist, db_new);
OPENMS_LOG_INFO <<
"Database entries (before / after): " << db.size() <<
" / " << db_new.size() << endl;
return EXECUTION_OK;
}
};
int main(
int argc,
const char** argv)
{
TOPPDatabaseFilter tool;
tool.main(argc, argv);
return 0;
}
#define OPENMS_LOG_FATAL_ERROR
Macro for fatal errors (processing stops) - includes file and line info.
Definition LogStream.h:542
#define OPENMS_LOG_INFO
Macro for information/status messages.
Definition LogStream.h:554
void loadIdentifications(const String &filename, std::vector< ProteinIdentification > &additional_proteins, PeptideIdentificationList &additional_peptides, const std::vector< FileTypes::Type > allowed_types={}, ProgressLogger::LogType log=ProgressLogger::NONE)
Loads an identification file into a proteinIdentifications and peptideIdentifications.
Open a pull request
Afterwards you can commit your changes to a new branch “feature/DatabaseFilter” of your OpenMS clone on github and submit a pull request on your github page. After a short review process by the OpenMS Team, the tool will be added the OpenMS Library.
Appendix
D-dimensional data points
The d-dimensional data points are needed in special cases only, e.g. in template classes that operate in any number of dimensions. The base class of the d-dimensional data points is DPeak. The methods to access the position are getPosition and setPosition. Note that the one-dimensional and two-dimensional data points also have the methods getPosition and setPosition. They are needed in order to be able to write algorithms that can operate on all data point types. It is, however, recommended not to use these members unless you really write such a generic algorithm.
OpenMS as external project
If OpenMS TOPP_tools and UTILS_tools are not sufficient for a certain scenario, you can either request changes to OpenMS or modify/extend your own fork of OpenMS. A third alternative is using OpenMS as a dependency while not touching OpenMS itself. Once you've finished your new tool, and it runs on the development machine, you're done. If you want to develop with OpenMS as external project have a look the example code ( /share/OpenMS/examples/external_code/).





